 I was going to start this blog with a witty reference to March Madness, but I don’t have a clue about college hoops. Instead, the only madness in March I have experienced is the release of the Meaningful Use Stage 3 proposed rule. To be fair, some aspects are good (such as simplifying the number of objectives), but some seem very ambitious.
I was going to start this blog with a witty reference to March Madness, but I don’t have a clue about college hoops. Instead, the only madness in March I have experienced is the release of the Meaningful Use Stage 3 proposed rule. To be fair, some aspects are good (such as simplifying the number of objectives), but some seem very ambitious.
On March 20, CMS released the 301-page Stage 3 proposed rule. Although the length of the document is slim compared to years past, it came with an companion document from the ONC that covers the proposed requirements for EHRs. For the sake of our attention span and blog length, I’m only going to cover the Eligible Professional (EP) objectives found in the proposed rule.
Getting Rid of the Stages
One way CMS is trying to simplify the program is by moving everyone into stage 3 in 2018 regardless of start date. In addition, if you are scheduled for Stage 3 in 2017, you are given the option to stay in Stage 2 for another year or move on to Stage 3.
In 2017, everyone (including first-year participants) will be in a full calendar year reporting period – except for those in year 1 with the Medicaid program. This move was done to help align the different CMS program reporting periods.
Stage of Meaningful Use Criteria by First Year
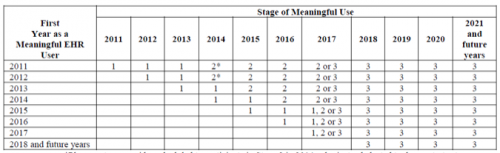
*A provider scheduled to participate in Stage 2 in 2014, who instead elected to demonstrate Stage 1 because of delays in availability of EHR technology certified to the 2014 Edition, is still considered a Stage 2 provider in 2014 despite the alternate demonstration of meaningful use. In 2015, all such providers are considered to be participating in their second year of Stage 2 of meaningful use.
Note: Information about the 2015 reporting period was not included in the Stage 3 proposed rule. It is set to be released in a separate ruling.
One of the biggest announcements in the document is that Stage 3 is expected to be the final stage for the Meaningful Use program. By incorporating portions of the prior stages into its requirement, CMS hopes to reduce “the complexity of the program” while keeping “the success of certain measures which are part of the meaningful use program to date” and also setting “a long-term, sustainable foundation based on a consolidated set of key advanced use objectives…”
The New Lineup
There are only eight objectives that make up the Stage 3 lineup:
- Protect Patient Health Information
- Electronic Prescribing (eRx)
- Clinical Decision Support (CDS)
- Computerized Provider Order Entry (CPOE)
- Patient Electronic Access to Health Information
- Coordination of Care through Patient Engagement
- Health Information Exchange (HIE)
- Public Health and Clinical Data Registry Reporting
Some objectives (or themes more like) are associated with multiple measures that are designed to “align with national health care quality improvement efforts,” “promote interoperability and health information exchange” and “focus on reducing cost, improving access and improving quality.”
In order to successfully demonstrate meaningful use, providers must meet all of the measures associated with an objective; however, some objectives provide flexibility by only requiring some of measures to be met.
Proposed Objective #1: Protect Patient Health Information
This repeat objective is described to “protect electronic protected health information (ePHI) created or maintained by the certified EHR technology (CEHRT) through the implementation of appropriate technical, administrative, and physical safeguards.”
Measure: The sole measure within this objective is to conduct or review a security risk analysis. Although this analysis is narrower than the HIPAA Security Rule 45 CFR 164.308(a)(1), it is still hefty and must be done annually. The ONC released a free Security Risk Assessment tool to help practices satisfy this measure.
Proposed Objective #2: Electronic Prescribing (eRx)
This is another repeat objective in the Stage 3 lineup requiring EPs to generate and transmit permissible prescriptions electronically.
Measure: More than 80 percent of all permissible prescriptions written by the EP are queried for a drug formulary and transmitted electronically using CEHRT.
Exclusions: Any EP who (1) writes fewer than 100 permissible prescriptions during the reporting period or (2) does not have a pharmacy within their organization and does not have any pharmacies that accept electronic prescriptions within 10 miles of the EP’s practice location.
Proposed Objective #3: Clinical Decision Support (CDS)
This is also a familiar objective requiring EPs to implement clinical decision support (CDS) interventions focused on improving performance on high-priority health conditions.
EPs must satisfy both measures in order to meet the objective:
Measure 1: Implement five CDS interventions related to four or more CQMs.
Measure 2: Enable and implement the functionality for drug-drug and drug-allergy interaction checks for the entire EHR reporting period.
Exclusion for Measure 2: Any EP who writes fewer than 100 medication orders during the EHR reporting period.
Proposed Objective #4: Computerized Provider Order Entry (CPOE)
This one is nearly identical to Stage 2—EPs must use CPOE for medication, laboratory and diagnostic imaging orders. Although similar to years past, CPOE has replaced the term “radiology” with “diagnostic imaging” to include other imaging tests (such as ultrasounds) along with upping the performance threshold. EPs must meet all of the following measures:
Measure 1: More than 80 percent of medication orders created by the EP during the EHR reporting period are recorded using CPOE.
Measure 2: More than 60 percent of laboratory orders created by the EP during the EHR reporting period are recorded using CPOE.
Measure 3: More than 60 percent of diagnostic imaging orders created by the EP during the EHR reporting period are recorded using CPOE.
Exclusion: EPs can be excluded from one or more of the measures if they write fewer than 100 of the type of order.
Proposed Objective #5: Patient Electronic Access to Health Information
The goal of this objective is to provide access for patients to view online, download, and transmit their health information, or retrieve their health information through an API, within 24 hours of its availability.
EPs must satisfy both measures in order to meet the objective:
Measure 1: For more than 80 percent of all unique patients seen by the EP, either:
- The patient (or patient-authorized rep) is provided access to view online, download, and transmit their health information within 24 hours of its availability; or
- The patient (or patient-authorized rep) is provided access to an ONC-certified API that can be used by third-party applications or devices to provide patients (or patient-authorized reps) access to their health information.
Measure 2: The EP must use clinically-relevant information from the EHR to identify patient-specific educational resources and provide electronic access to those materials to more than 35 percent of unique patients seen by the EP during the EHR reporting period.
Exclusion: An EP that has no office visits during the EHR reporting period or any EP that conducts 50 percent or more of his or her patient encounters in a county that does not have 50 percent or more of its housing units with 4Mbps broadband availability.
One significant change to the second measure is the removal of paper education materials. CMS states that EPs can (and still should if it makes sense) deliver patient education materials via paper, but if they do, these transactions cannot count towards the numerator.
Proposed Objective #6: Coordination of Care through Patient Engagement
This is the objective that may make or break most providers’ ability to be successful. EPs will be required to communicate (via EHR) with patients (or their authorized reps) about the patient’s care – and it can’t just be test messages and appointment reminders.
Providers do have some flexibility in this category by only needing to successfully meet two of the three measures.
Measure 1: During the EHR reporting period, more than 25 percent of all unique patients seen by the EP actively engage with the EHR made accessible by the provider.
An EP may meet the measure by either:
- More than 25 percent of all unique patients (or patient-authorized reps) seen by the EP view, download or transmit to a third party their health information; or
- More than 25 percent of all unique patients (or patient-authorized reps) seen by the EP access their health information through the use of an ONC-certified API that can be used by third-party applications or devices.
Measure 2: For more than 35 percent of all unique patients seen by the EP during the EHR reporting period, a secure message was sent to the patient (or the patient’s authorized rep), or in response to a secure message sent by the patient (or the patient’s authorized rep). Unlike in Stage 2, a message initiated by the provider will count towards this measure.
The thresholds for both measures seem to be quite ambitious. Although national benchmarking for these measures in Stage 2 are not yet available, it seems even the current Stage 2 thresholds for these measures are very difficult to meet.
Measure 3: Patient-generated health data or data from a non-clinical setting is incorporated into the certified EHR technology for more than 15 percent of all unique patients seen by the EP during the EHR reporting period.
This last measure is new in Stage 3 and still quite vague. This measure is supposed to help “support the capture of patient-generated health data” which may result from patient self-monitoring of their health.
Proposed Objective #7: Health Information Exchange (HIE)
This objective is another huge hurdle that really pushes EHRs and EPs to become more interoperable. EPs would only be required to successfully report two of the three proposed measures to meet the objective.
Measure 1: For more than 50 percent of transitions of care and referrals, the EP that transitions or refers their patient to another setting of care or provider of care, both:
- Creates a summary of care record using CEHRT, and
- Electronically exchanges the summary of care record.
Exclusion for Measure 1: An EP who neither transfers a patient to another setting nor refers a patient to another provider during the EHR reporting period OR any EP that conducts 50 percent or more of his or her patient encounters in a county that does not have 50 percent or more of its housing units with 4Mbps broadband availability.
Measure 2: For more than 40 percent of transitions or referrals received and patient encounters in which the provider has never before encountered the patient, the EP incorporates into their EHR an electronic summary of care document from a different source.
Exclusion for Measure 2: Any EP for whom the total of transitions or referrals received and patient encounters in which the provider has never before encountered the patient, is fewer than 100 during the EHR reporting period is excluded from this measure. Also, an EP that conducts 50 percent or more of his or her patient encounters in a county that does not have 50 percent or more of its housing units with 4Mbps broadband availability.
Measure 3: For more than 80 percent of transitions or referrals received and patient encounters in which the provider has never before encountered the patient, the EP performs a clinical information reconciliation. The provider must implement a clinical information reconciliation for the following three clinical information sets:
- Medication—Review of the patient’s medication, including the name, dosage, frequency, and route of each medication.
- Medication allergy—Review of the patient’s known allergic medications.
- Current Problem list—Review of the patient’s current and active diagnoses.
Proposed Objective #8: Public Health and Clinical Data Registry Reporting
Last, but definitely not least, this objective builds on the requirements from Stage 2. The proposed objective requires the EP to actively engage with a public health agency (PHA) or clinical data registry (CDR) to submit data via the EP’s EHR.
There are a total of six possible measures. An EP must choose any combination of three measures.
Measure 1: Immunization Registry Reporting—The EP is in active engagement with a PHA to submit immunization data while also receiving data from the immunization information systems (IIS).
Exclusion for Measure 1: The EP (1) does not administer immunizations; (2) operates in a jurisdiction for which no IIS is capable of accepting a certified message at the start of the EHR reporting period; or (3) operates in a jurisdiction where no IIS has declared readiness to receive immunization data at the start of the EHR reporting period.
Measure 2: Syndromic Surveillance Reporting—The EP is in active engagement with a PHA to submit syndromic surveillance data from a non-urgent care ambulatory setting.
Exclusion: The EP (1) does not diagnose or directly treat any disease or condition associated with a syndromic surveillance system in their jurisdiction; (2) operates in a jurisdiction for which no PHA is capable of receiving electronic syndromic surveillance data; or (3) operates in a jurisdiction where no PHA has declared readiness to receive syndromic surveillance data from EPs at the start of the EHR reporting period.
Measure 3: Case Reporting—The EP is in active engagement with a PHA to submit case reporting of reportable conditions.
Exclusion for Measure 3: Any EP meeting one or more of the following criteria may be excluded from the case reporting measure if the EP (1) does not treat or diagnose any reportable diseases for which data is collected by their jurisdiction’s reportable disease system during the EHR reporting period; (2) operates in a jurisdiction for which no PHA is capable of receiving electronic case reporting data in the specific standards required; or (3) operates in a jurisdiction where no PHA has declared readiness to receive electronic case reporting data at the start of the EHR reporting period.
Measure 4: Public Health Registry Reporting—The EP is in active engagement with a public health agency to submit data to public health registries.
Exclusion for Measure 4: Any EP meeting at least one of the following criteria may be excluded from the public health registry reporting measure if the EP (1) does not diagnose or directly treat any disease or condition associated with a PHA in their jurisdiction during the EHR reporting period; (2) operates in a jurisdiction for which no PHA is capable of accepting electronic registry transactions in the specific standards; or (3) operates in a jurisdiction where no public health registry for which the EP is eligible has declared readiness to receive electronic registry transactions at the beginning of the EHR reporting period.
Measure 5: Clinical Data Registry Reporting (CDR)—The EP is in active engagement to submit data to a clinical data registry.
Exclusions for Measure 5: Any EP meeting at least one of the following criteria may be excluded from the clinical data registry reporting measure if the EP (1) does not diagnose or directly treat any disease or condition associated with a CDR in their jurisdiction during the EHR reporting period; (2) operates in a jurisdiction for which CDR is capable of accepting electronic registry transactions in the specific standards; or (3) operates in a jurisdiction where no CDR for which the EP is eligible has declared readiness to receive electronic registry transactions at the beginning of the EHR reporting period.
Measure 6: Electronic Reportable Laboratory Result Reporting—This measure is only applicable for eligible hospitals.
In addition to the objectives, EPs must still report CQMs (but I’ll spare you those proposed details for another day).
Hardship Exceptions?
After reading most of this dissertation, you’re probably asking yourself what hardship exceptions have been proposed for Stage 3. Thankfully, the same hardship exceptions from Stage 2 are being proposed:
- The lack of availability of Internet access or barriers to obtain IT infrastructure.
- A time-limited exception for newly practicing EPs.
- Unforeseen circumstances such as natural disasters.
- Lastly, a nephrologist favorite—exceptions due to a combination of clinical features limiting a provider’s interaction with patients or, if the EP practices at multiple locations, lack of control over the availability of CEHRT at practice locations constituting 50 percent or more of their encounters.
What are your thoughts on the proposed Stage 3 ruling? Did CMS create a simple and flexible program for the future or are you swimming in confusion? Let us know what you would change by leaving a comment below!
 Diana Strubler, Senior Product Analyst, Health IT Standards, joined Acumen in 2010 as an EHR trainer then quickly moved into the role of certification and health IT standards subject matter expert. She has successfully led Acumen through three certifications while also guiding our company and customers through the world of Meaningful Use, ICD-10 and PQRS.
Diana Strubler, Senior Product Analyst, Health IT Standards, joined Acumen in 2010 as an EHR trainer then quickly moved into the role of certification and health IT standards subject matter expert. She has successfully led Acumen through three certifications while also guiding our company and customers through the world of Meaningful Use, ICD-10 and PQRS.



Leave a Reply