 This July 4th weekend has been all flags and fireworks in red, white, and blue. What better time to think about our Federal Government and the laws of the land? Jiggery-pokery and applesauce aside, the Medicare-funded ESRD program has many rules and regulations, most of which are preceded by the Centers for Medicare and Medicaid Services (CMS) publication of “proposed rules.” A proposed rule is an official government agency interpretation of legislation that has the full force of the law. Let’s look at some proposed updates for the ESRD Quality Incentive Program (QIP).
This July 4th weekend has been all flags and fireworks in red, white, and blue. What better time to think about our Federal Government and the laws of the land? Jiggery-pokery and applesauce aside, the Medicare-funded ESRD program has many rules and regulations, most of which are preceded by the Centers for Medicare and Medicaid Services (CMS) publication of “proposed rules.” A proposed rule is an official government agency interpretation of legislation that has the full force of the law. Let’s look at some proposed updates for the ESRD Quality Incentive Program (QIP).
On June 26, CMS published updates to policies and payment rates for dialysis in calendar year 2016, which included proposed changes to the ESRD QIP. This CMS document includes proposed changes to the base reimbursement or bundled rate for dialysis and other reimbursements as required by law. Other issues including payment for oral-only ESRD medications and payment adjustments for co-morbidities are also addressed. The last section of this fact sheet on the proposed rule addresses the ESRD QIP payment year 2019.
A few reminders about QIP
Based on the national healthcare quality strategy the Federal Government is focused on 6 domains of healthcare quality measurement:
- Care coordination
- Population health
- Patient safety
- Affordability
- For individuals, families, employers, and the government
- Patient and family engagement
- Treatment and prevention of chronic diseases
- Best practices
The ESRD QIP is a poster-child program that defines quality of care, measures it, and penalizes providers who do not deliver quality care. To be clear, the penalty for not meeting the quality standard is the “incentive” part of the Quality Incentive Program. The structure for this incentive program was created by Section 1881(h) of the Social Security Act and Section 153(c) of the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA). CMS articulates the QIP program purpose as “promoting patient health by providing a financial incentive for renal dialysis facilities to deliver high-quality patient care.” The Social Security Act gives CMS the opportunity to use Medicare payment reductions to incent improvement in care delivery.
QIP meets all of the CMS objectives for a value-based purchasing program including:
- Reporting of evidence-based clinical care measures
- Transparency of performance
- Payment models that reinforce performance improvement
- Incorporating Meaningful Use strategies for healthcare information
- Achieving healthcare equity to eliminate healthcare disparities
MIPPA specifically gives CMS and the Secretary of Health and Human Services (HHS) the authority to:
- Select quality measures
- Establish performance standards
- Specify the performance period that is tied to the Payment Year (PY)
- Assess the facility Total Performance Score (TPS)
- Determine the payment reduction for each facility based on the TPS
- Publicly report the results
The impact on dialysis facilities
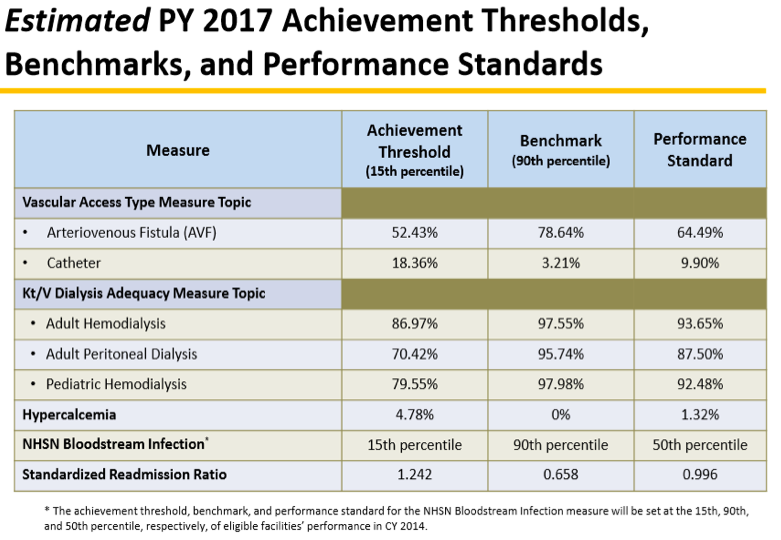
This year our dialysis facility performance is being measured for PY 2017. 75% of the TPS for 2015 is calculated from 8 clinical measures:
- AVF rates
- Catheters >/= 90 days
- Kt/V dialysis adequacy measure in adult hemodialysis (HD)
- Kt/V dialysis adequacy measure in adult peritoneal dialysis (PD)
- Kt/V dialysis adequacy measure in pediatric HD
- Hypercalcemia (proportion of patient-months with 3-month rolling average of total uncorrected serum calcium greater than 10.2 mg/dL)
- NHSN blood stream infections (BSI)
- Standardized readmission ratio (number of observed unplanned 30-day hospital readmissions to the number of expected unplanned 30-day hospital readmissions)
25% of the TPS is based on reporting of 3 measures:
- In-center HD Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey results
- Mineral metabolism (report either serum or plasma phosphorus)
- Anemia management (report ESA dosing, hemoglobin, and hematocrit at least once per month)
CMS estimates the performance required to determine possible penalties in the PY. Here are the estimates for 2015 performance to be penalized in PY 2017:
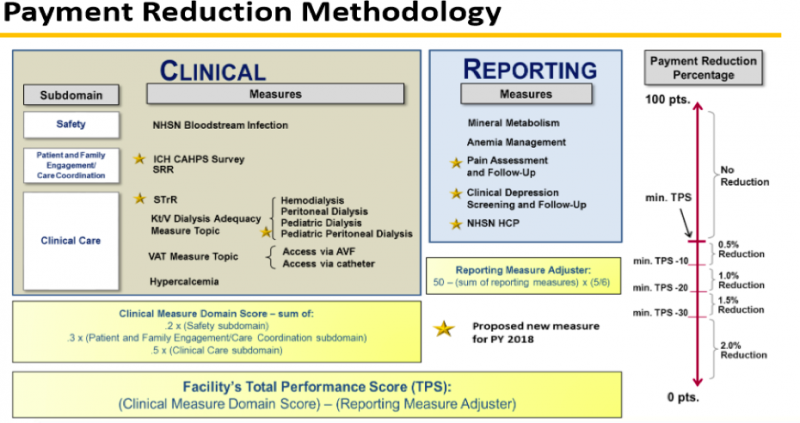
The QIP performance measures for PY 2018/performance year 2016 are set with 11 clinical performance measures and 5 reporting measures. Here is the CMS table showing how these are measured and scored:
One notable change related to QIP performance 2016/PY 2018 is the reporting of Healthcare Personnel influenza vaccination (NHSN HCP). This is a reporting measure next year, but will surely become a true performance measure in the future. Patient influenza vaccination reporting is part of the performance 2017/ PY 2019 proposed rule. Other PY 2019 reporting measures include an ultrafiltration rate reported for every qualifying patient, clinical depression screening and follow up, and pain assessment and follow up as documented in CROWNWeb.
The PY 2019 proposed rule also includes the controversial Standardized Transfusion Ration (STrR). This clinical measure is a ratio of number of observed eligible red blood cell transfusion events occurring in patients dialyzing at a facility to the number of eligible transfusions that would be expected from a predictive model that accounts for patient characteristics within each facility.
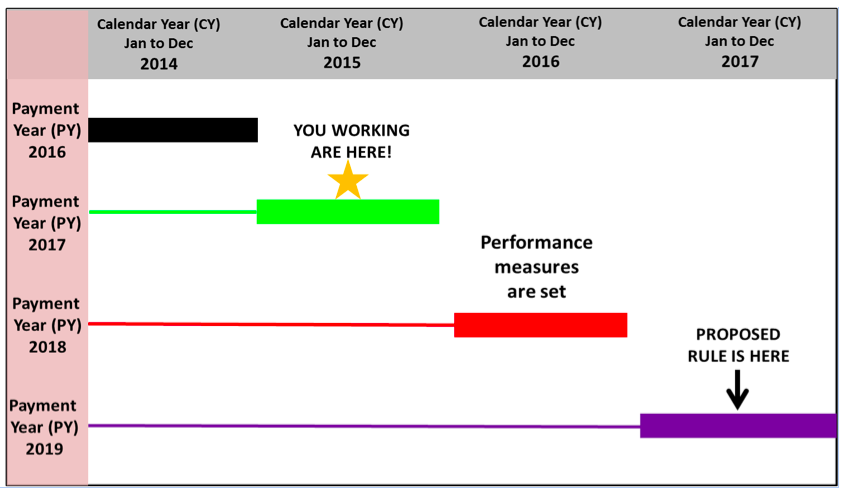
The impact of a 4-year cycle
While in some ways following a 4-year cycle seems to be planning ahead, it does mean that fixed measures in 2017 may or may not be state of the science 2 years from now. The measures risk lagging behind what is clinically best practice, but we do have fair warning of what the rules will be. The proposed rule for the measures is published 2 years before the measurement/performance year and the PY is 2 years later. Here is the schedule:
Dialysis facility performance in 2015 will be penalized in PY 2017. The proposed rule to be finalized in 2015 will set performance measures collected in 2017 and penalized in reimbursement in PY 2019. CMS provides information about the QIP details through Medicare Learning Network (MLN) National Provider calls, which are open to ESRD-facility stakeholders including nephrologists. A QIP PY 2019 call is scheduled on July 29 from 2:00–3:30 pm EST. Information about participation can be found on the CMS website. There are plenty of rules, but you can exercise your right to contribute to the discussion.
 Dugan Maddux, MD, FACP, is the Vice President for CKD Initiatives for FMC-NA. Before her foray into the business side of medicine, Dr. Maddux spent 18 years practicing nephrology in Danville, Virginia. During this time, she and her husband, Dr. Frank Maddux, developed a nephrology-focused Electronic Health Record. She and Frank also developed Voice Expeditions, which features the Nephrology Oral History project, a collection of interviews of the early dialysis pioneers.
Dugan Maddux, MD, FACP, is the Vice President for CKD Initiatives for FMC-NA. Before her foray into the business side of medicine, Dr. Maddux spent 18 years practicing nephrology in Danville, Virginia. During this time, she and her husband, Dr. Frank Maddux, developed a nephrology-focused Electronic Health Record. She and Frank also developed Voice Expeditions, which features the Nephrology Oral History project, a collection of interviews of the early dialysis pioneers.



Leave a Reply